For those who are prescribed flower, we recommend that upon receipt of your meds, you tip the cannabis out onto a plate and quickly inspect the flower for any issues.
Thanks to patient Mel B. for helping to work through this process with us (and providing the images).
Far out…there’s foreign matter in my medicine
Medical cannabis is different from most other ‘traditional’ medications in the fact that it doesn’t usually have many foreign chemicals or additives. In the case of cannabis flower, you are getting a very natural medication that is organic material. While medical cannabis in all forms goes through various quality tests along the journey from seed to patient, there is still a chance that the product you get will not be perfect.
This article is to explain the process for patients with a certain type of problem. If you find foreign matter or mould in your medicine or find a product defect this article is for you. This process covers:
- Mould in flower
- Small foreign objects in flower or oil
- Faulty or broken medical devices
- Missing or broken seals on products
There may be other issues that fall into this category as well.
This article is not for people who have received a batch of medicine, particularly oil, and the colour, taste or smell is different. It’s also not for a patient who has noticed that a new batch of medication isn’t working as well as the last batch. We’ll have more on those topics in the near future.
The process we’ll explain below was tested with a patient who found some sort of larvae (bug egg) in their cannabis flower. Within days of following this process, the patient had their medication replaced and a TGA incident number provided to them by their doctor/product manufacturer. While finding the foreign matter wasn’t good, the outcome for the patient was ideal in terms of support and a solution.
It’s also important to note that this defect was not ‘unsafe’. All flower goes through a sterilisation process, which, in this case neutralised the foreign matter.
A final note on when this process should be used. With things like a broken seal or a missing syringe, you need to use your judgement. When there is a minor product defect, going to your doctor and the manufacturer may suffice.
The TGA is there to protect patients. They do this by making sure suppliers and manufacturers are following the required processes and taking appropriate action on all quality issues. So, if you think that your problem can be solved by your doctor or the manufacturer/supplier start there.
This article contains the following sections:
How foreign matter ends up in cannabis
We have three major categories of cannabis products in Australia:
- Australian-grown and manufactured.
- Imported materials and manufactured in Australia.
- Products (and plant) imported in final form from overseas.
And, there are companies that choose to go through multiple of the above pathways. For example, there are some companies that are mostly Australian grown and manufactured but supplement their products with overseas resins when supply is low.
To be clear, products that are imported or products that contain imported plant matter are not necessarily bad. In many cases they are excellent. However, all Australian products must meet TGO 93 requirements, and the TGA audits local products from seed to patient.
Products and biomass coming from overseas declare meeting the TGO93 criteria, but the initial stages of their process are not audited. We know that local cultivators and manufacturers are audited by the TGA regularly. Imported products, however, may be audited less frequently and audits to confirm that all aspects of imported products meet TGO93 seem to be rare.
Our regulations also require local providers to meet GMP (process standards) which are very strict. Overseas imports don’t have the same requirement.
Regardless of the requirements, the TGA does allow for some error in the process. That’s because it’s not possible to have perfection. Because our cannabis products are coming from all over the world and go through so many steps, from cultivation to final product, there is a chance you may find foreign matter or a defect with your cannabis product.
In flower products we’ve heard of mould or larvae from insects; in oil we’ve heard of small pieces of debris floating around other anomalies.
The Process
Firstly, if you’re unlucky enough to find foreign matter in your medicine, we’re sorry that you have to deal with this inconvenience. The good news is that dealing with this problem is not as difficult as you may think. There are three steps to this process:
- Document the issue thoroughly (with photos)
- Email your doctor and the manufacturer (or supplier)
- Contact the TGA
At first glance, you may think that contacting three different entities for this issue is overkill. However, there are important reasons for contacting each party. It’s important to contact your doctor because you are no longer able to take your medication. The doctor will need to give you some advice and solutions for how to manage.
The manufacturer needs to know that this issue has happened for quality and regulatory purposes and because they need to organise new medication for you. Finally, contacting the TGA is critical because they keep a log of quality breaches and will review the issue with the manufacturer.
Providing this information to the TGA also means that the regulatory body can dive deeper into your medicine batch with the manufacturer if necessary. The problem you have found may be impacting other patients.
Documenting the issue
Documenting the problem thoroughly is critical. It shows all parties involved that you’re taking the issue seriously and that you’ve done your best to mitigate any further contamination of the product.
When documenting your findings, you’ll need:
- Photos of the foreign matter (along with your packaging).
- Product info and a detailed timeline of how long you’ve had the product/received it to when you noticed the foreign matter.
- If you’ve had the product for a while, how much did you consume and how were you storing it?
- Steps you took after finding the foreign matter.
- What you’d like to get out of this process.
Photos
Photos of the product and foreign matter are very helpful for the manufacturer and TGA (if requested) so that they can get an initial understanding of what the problem actually is. Don’t just take a photo of oil or flower alone on a table. Do your best to take a photo of the packaging along with the actual product and foreign matter. Here’s an example:

Product info and timeline
There are a number of reasons it’s important to detail the timeline. Firstly, if the product has an issue and you’ve consumed a lot of it, your doctor may recommend that you book an appointment. Secondly, if you report the issue shortly after finding it, you’re more likely to get a positive response from the TGA and manufacturer.
Finally, the sooner you find it and report it, the better it is for all patients receiving that medication. Depending on the severity of the problem, other patients might be affected too.
Steps you took after finding the foreign matter.
While the manufacturer’s goal should be to help you when learning about the issue, you want to make sure you’ve protected yourself from being seen as adding to the problem. So, you’ll want to explain how you were storing the product prior to finding the foreign matter and once you found it.
Be clear and precise in the steps you took to mitigate any further product contamination.
Sample Timeline & Steps Table example:
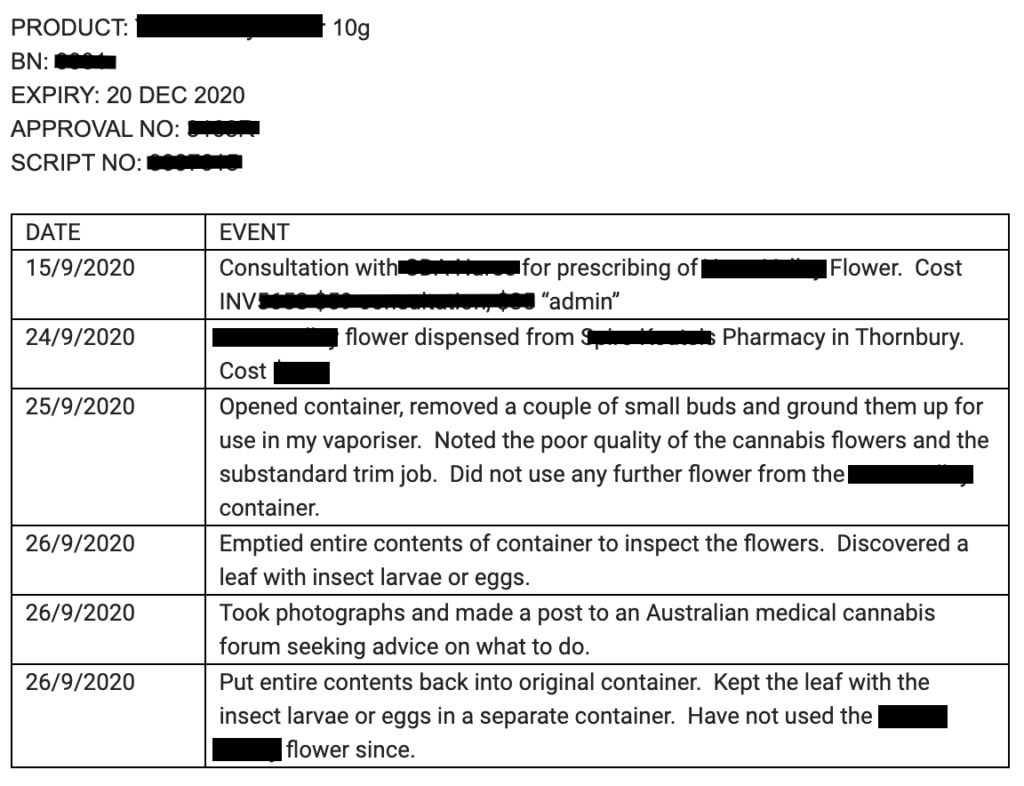
What you’d like to get out of this process.
While the obvious goals are to get yourself a new, non-contaminated medication and to make sure that other patients are safe and don’t have the same quality issue, you may also want other specific outcomes.
Perhaps you’re no longer comfortable continuing with the product and as a result, you’d like your money back. Perhaps you’d simply like to raise concern about others who may have the product. As a patient, you might like a phone call from someone at the manufacturer to explain how your matter is being handled.
While many points above seem obvious, be clear about exactly what you want from both your doctor and the supplier.
Finally, it’s important to request your TGA Adverse Event Report and TGA identifier number. You can request this from both the manufacturer and the TGA. This number is your receipt of your complaint.
Example Email Template
Here’s an example of a timeline and the information required by the TGA which you should also send to your doctor and manufacturer:
Sample email:
Subject: Product Name – Batch # – Title of Issue (ie. Oil Contained Foreign Matter Upon Opening)
Dear Dr X and Manufacturer/Supplier Name,
I hope you are well.
My name is insert name and I’m a patient of Dr X. On insert day, the insert date, I received insert product name, batch: insert batch number. When I opened the packaging I inspected the flower/oil. Upon inspection, I found insert problem. Attached is an image of the insert problem with my packaging.
I’ve detailed the process I followed since finding the insert problem in the table below. I’ve done research on the appropriate steps to inform all parties of this adverse event and am writing to make a formal record of this issue.
I will also be contacting the TGA today to advise them of this product quality/safety issue. It is important not only for me but for other medical cannabis patients in Australia.
I would like….insert the outcome you would like. Please also provide me with my TGA Adverse Event Report and TGA identifier number.
Finally, I would like a written response to this email no later than close of business Insert Date. Thank you for your help.
Kind regards,
Name
Insert contact number
Once you have this information written out clearly, it’s time to contact your doctor, the manufacturer and the TGA.
Contacting your doctor and the manufacturer
Ideally, you’ll be able to contact your doctor and the manufacturer on the same email. Doing so ensures that both parties learn about the incident at the same time and can work together to resolve the issue.
IMPORTANT: In a case where the manufacturer only has a contact form on their website, provide the manufacturer with your doctor’s contact details: name, email address and phone number.
Contact the TGA
Now you’ll want to make sure that you give the TGA pretty much all the same exact information with slightly different context.
You contact the TGA via their online form.
For the “Name of the product of concern” make sure you put in the full product and brand name:
- Example: honahlee – cbd 90 – medical cannabis – found mould in oil
For the details of the problem, give them the exact same product details and timeline that you give the doctor and manufacturer
From here it’s a follow-up and waiting game.
Final Actions
At this stage, you will likely want to call your doctor. We recommend doing so after you’ve sent the email and details because you want to have a record of everything that happened in this process.
Regardless of whether the doctor, manufacturer or TGA follows up via phone, it’s important you keep written records of everything. After a phone call, make notes and send a recap email to the contact.
The bottom line is that all three parties should have a patient-first attitude and be helpful and kind throughout the entire process.
When this process was first followed by a patient we worked with, the patient’s issue was resolved within 48 hours. The manufacturer/doctor provided them with new (different) products as requested and there was no charge for the new product to make up for the contaminated product.
The manufacturer and doctor were the main contacts for the patient. The manufacturer provided the patient with the TGA Adverse Event Report. While everything was resolved, the TGA never reached out to the patient, so don’t expect to hear from the TGA, only the manufacturer and doctor.
If you go through this process and still have trouble, please don’t hesitate to reach out to the honahlee team for further support.


