Important: The information in this article is not legal advice and is not provided by lawyers. If you have questions about the law, please consult a legal practitioner.
When the Therapeutics Goods Administration (TGA) changed the prescriber authorisation process from product-specific to category-based in 2021, patients started reporting access to Generic or Open Scripts. This ‘new’ script format was a topic of contention, with some prescribers and pharmacists adamant that patients could access this type of script. For over a year, industry bodies, health professionals and patients sent requests to the TGA and State Health Departments. No one ever came back with a straight answer.
The concept of the Open Script is akin to the idea of conventional biosimilar medication substitution or active ingredient prescribing, which has been a part of conventional medicine for years.
In this article, we’ll disclose some of the information the TGA and state bodies have provided to help answer the most frequently asked questions about the Generic or Open Medicinal Cannabis Script. To learn more about the changes from product-specific prescriber authorisations to category authorisations, check out our TGA Category Approval Changes article. Please note that we use the term Generic and Open Script interchangeably. We’ll use the term Open Script for the remainder of this article.
Here’s what the article covers:
- What is a Generic or Open Script?
- Can doctors/prescribers write an Open Script and will pharmacies fulfil them?
- Can I choose my own product with an Open Script?
- Where can I fulfil an Open Script?
- What we know about whether Open Scripts are valid scripts
- TGA Guidance
- State Health Dept Responses
- The conventional biosimilar medication substitution framework
- An interpretation of guidance that allows for open scripts
- Conclusion
What is a Generic or Open Script for medicinal cannabis?
Open Scripts are prescriptions (physical paper or eScripts) written with the active product ingredients of the medicinal cannabis products (ie Cannabidiol and/or Tetrahydrocannabinol), a specific value or range of those active ingredients and the plant species (indica or sativa) as well as other information required to be on the prescription.
Here’s an example of what the information on an Open Script could look like:
- 17-22% tetrahydrocannabinol, <2% cannabidiol, 10g, Sativa Dominant, herb, dried for inhalation
- 24% THC, <2% CBD, 15g, Hybrid, herb, dried for inhalation
For contrast, a conventional script would be written as follows:
- Brand name, Product Name, 120 capsules, 25mg CBD, 2mg THC
- Brand name, Product Name, 10g Dried Flower, <1% CBD, 20% THC
The scripts may be written slightly differently. With a conventional prescription, the prescriber has selected the product in consultation with the patient. With an Open Script, the patient works with the pharmacist to choose a product or different products each time the patient fulfils the prescription.
Can doctors/prescribers write an Open Script and will pharmacies fulfil them?
Yes, some prescribers write Open Scripts. We know this because patients regularly report on social media and via other channels that their prescriber has written them an Open Script. We also speak with pharmacies that are comfortable dispensing Open Scripts.
Whether these scripts are valid prescriptions is still open for debate. However, we have been told by prescribers and pharmacists that their respective governing bodies have audited them and have not been punished for writing or fulfilling these types of prescriptions.
Can I choose my own product with an Open Script?
The idea behind an Open Script is that the patient works with the pharmacist to choose the product. So, you should not approach a pharmacy or pharmacist demanding a specific product. The pharmacist should consult with you about the products they feel are best and the products you think would work best for you and come to an agreement.
Then, the next time you fill your prescription, you would either have the same product or if you and the pharmacist felt there was a potentially better option, the pharmacist may dispense that product instead.
Where can I fulfil an Open Script?
Just as there are prescribers across Australia writing Open Scripts, some pharmacies are happy to dispense Open Scripts.
If you have an eScript and are interested in finding a pharmacy that is comfortable dispensing open scripts, head to Scripts by honahlee and use the Generic Script filter after uploading your eScript. You’ll find a list of pharmacies that are comfortable dispensing Generic/Open Scripts and won’t get locked into the system.
What we know about whether Open Scripts are valid scripts
The honahlee team has done a lot of research into whether the Open Script is valid. Initially, it seemed very clear that Open Scripts were not valid. As we dug further and started speaking to pharmacies dispensing and prescribers writing Open Scripts, the waters were muddied yet again. Here’s what we’ve found.
TGA guidance on writing Open Scripts
It’s important to remember that the TGA does not regulate prescription writing or the filling of prescriptions. These are duties of the State Health Departments and bodies/organisations that oversee health professionals and pharmacists.
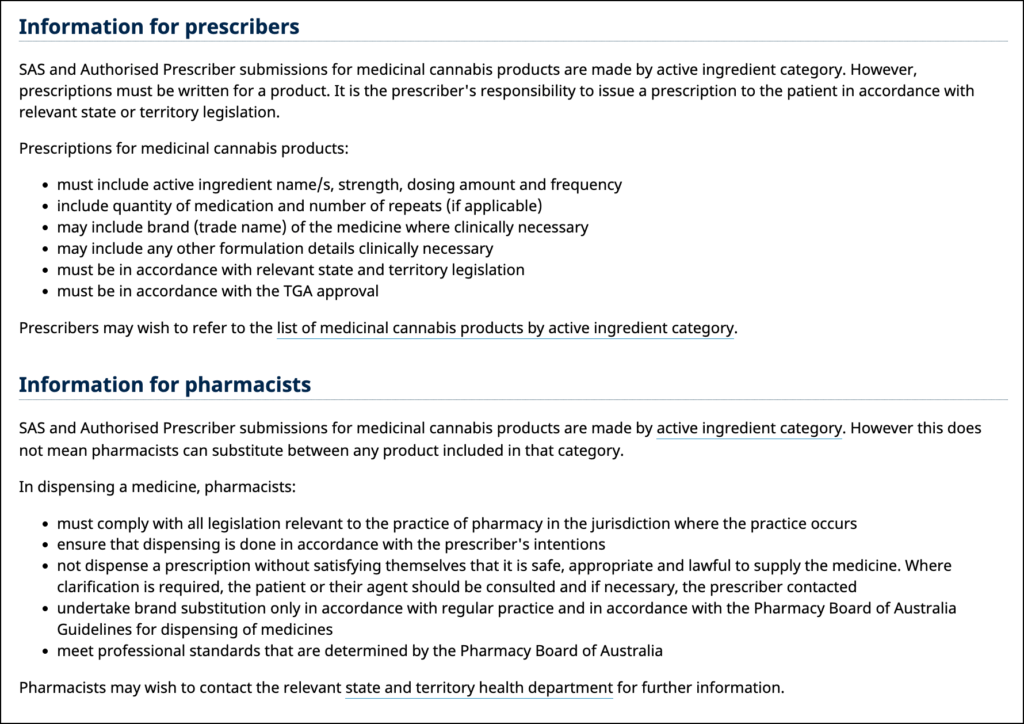
TGA updated their website with the information in the image above. While the TGA doesn’t explicitly state that Open Scripts may not be written, they are clear about a few things:
- Prescriptions must be written for a product.
- Prescriptions must have the strength written.
- Pharmacists may not just substitute between any products in a category.
- Pharmacists must undertake brand substitution in accordance with PBA Guidelines.
Based on the information above, it is safe to say that the following rules apply:
- A script that only lists a range of CBD:THC or a category is not a “prescription for a product”.
- A script that does not list a brand name would need to list the exact strength and CBD/THC content.
- A pharmacist cannot just choose or substitute products – they must have a reason.
These factors alone make it seem like an Open Script is not a valid prescription.
State Health Departments guidance on writing Open Scripts
When we launched our Scripts by honahlee platform, we contacted each State Health Department (SHD) to get their opinion on Open Scripts. We gave each department examples similar to the following:
- 17-22% tetrahydrocannabinol, <2% cannabidiol, 10g, Sativa Dominant, herb, dried for inhalation
All SHDs said scripts written in the above format would not be valid prescriptions. Each SHD had slightly different reasons. However, they all pointed back to their Medicines and Poisons regulations and explained how they were interpreting those regulations.
A couple of the SHDs said that a prescription written in the following format would be acceptable:
- 24% THC, <2% CBD, 15g, Hybrid, herb, dried for inhalation
The difference between the two is that the first example has a range (all SHDs said not valid), and the second has a specific value but no brand or product name (some states said valid).
The conventional biosimilar medication substitution framework
The substitution of brands in conventional medications has been going on for a long time. Most patients have gone to their pharmacy to drop off or pick up a script and been asked if they’d like the cheaper or generic brand. If this has been happening with other medications, why is it so complicated with cannabis?
The Department of Health website says the following about biosimilar medicines:
- Biosimilar medicines are highly similar versions of an already registered biological medicine (the reference biological medicine).
- The minor differences between reference and biosimilar medicines do not affect the safety, effectiveness or quality of the biosimilar medicine.
- The medicines have been assessed to have no clinically meaningful differences and are therapeutically equivalent.
- A medicine cannot be identified as a biosimilar medicine until it is evaluated by the Therapeutic Goods Administration (TGA) and shown to be as safe and effective as the reference biological medicine.
Medicinal cannabis is an unapproved medication, so prescribers must use the SAS and other special pathways. As an unapproved medication, cannabis has not been evaluated to have biosimilar equivalents. The TGA also keeps a list of approved biosimilar medications, and no cannabis-related products are on the list.
How prescribers & pharmacists justifying Open Scripts
Reminder: The information in this article is not legal advice and is not being provided by lawyers. If you have questions about the law, please consult a legal practitioner.
Up to this point, we’ve presented information that leads us to believe that Open Scripts, specifically those with a range, are not valid prescriptions, and those with no brand but a specific Cannabinoid Content are valid in some states.
So, how are prescribers and pharmacists justifying the Open Script?
From our research, which includes conversations with prescribers and pharmacists, there is a small consensus that the Department of Health and Aged Care guidance allows for Open Scripts.
As mentioned earlier in this article, there are two types of Open Scripts being written:
- One that provides a range for cannabinoid percentage (ie 17-22% tetrahydrocannabinol, <2% cannabidiol, 10g, Sativa Dominant, herb, dried for inhalation)
- One with a specific cannabinoid percentage (ie 24% THC, <2% CBD, 15g, Hybrid, herb, dried for inhalation)
Based on the information we’ve been provided, the second seems more likely to be a valid script than the first due to the fact that it doesn’t have a range of cannabinoids. And, we’ve not found any other medication where a health professional can write a prescription for a range of an active ingredient. However, there are still prescribers and pharmacists arguing that a script with a range is valid.
While we once believed that it was obvious that Open Scripts are not valid prescriptions, this new information leads us to think that Open Scripts may be valid prescriptions.
Conclusion
To explain the current interpretations of the Generic/Open Scripts, we’ve provided information we’ve seen from the TGA and been told by the State Health Departments. We’ve also provided an overview of what prescribers and pharmacists actively engaging with Open Scripts have shared.
We cannot say whether these scripts are valid, but we know that patients Australia-wide have them and need access to their medication. We’ll continue to investigate the Open Script until we have a finite answer and will update this article as we learn more.
Until then, we’ll work with pharmacies that can help patients access their medication with Open Scripts via Scripts by honahlee.


