Video Timings:
00:30 | TGA guidelines: What they are and why they exist
02:45 | Permitted Education / Advertising
03:30 | An example of balanced and factual cannabis education
04:15 | Education / Advertising not permitted
04:30 | Definition of advertising (TGA Guidelines) and interpretation
06:32 | Advertising of services vs products
08:44 | How these guidelines impact patients
11:40 | The importance of education and some educational resources
14:15 | How these guidelines impact industry
17:30 | Questions you should ask your prescriber (and the reasons why)
18:30 | How the TGA enforces these guidelines
20:20 | How the TGA finds those doing non-permitted advertising
22:15 | How reactive responses from the TGA hurt industry and patients
24:21 | Possible solutions part 1
28:57 | Do the guidelines make sense for the industry?
30:58 | Possible solutions part 2
34:48 | Enforcing the guidelines for the most obvious breaches
36:06 | Takeaways
TGA advertising guidelines & their importance
At honahlee, we are values-driven and the patient/consumer are the focus of everything we do. We value transparency, education, diversity and community. While this article and accompanying video may come across as having a go at certain industry players, it is not. We are simply shining a light on issues and conflicts of interest that the cannabis community must know about in order to make educated decisions. Thank you to our partners who have helped us create this and many other pieces of content. Your support has helped us better inform and educate the Australian community about cannabis.
The advertising of pharmaceuticals to patients and consumers in Australia is not allowed. These rules were put in place to help protect the general public against false claims, and to reduce the amount patients and consumers can be influenced by information about drugs.
While many patients may not see the rules as important or having an impact on them directly, these guidelines and the operation of companies outside of these guidelines have a big impact on the patient population. The problem with these guidelines is that they blur education and advertising. There are two things that are very clear.
The first is that patients need more factual and unbiased cannabis education. The second is that patients need to know that some clinics and product companies have conflicts of interest in the way they advertise and supply their products. If patients don’t have all the information they will trust whatever they discover on the web and social media, and make lesser informed decisions which may not be best for their health.
If you’re a patient reading this article and are short on time, we recommend you read the section that covers how the guidelines impact patients.
The goal of this article is to give patients an understanding of how the TGA guidelines work, how they impact the broader cannabis community, and to start a conversation about what information patients want and need to improve the cannabis space. In this article we cover:
- TGA guidelines: What they are and why they exist
- Permitted education and advertising
- Prohibited education and advertising
- How these guidelines impact patients
- How these guidelines impact the industry
- How the TGA enforces the guidelines
- Should cannabis be treated like a pharmaceutical?
- Do the guidelines work? Do they need to change?
TGA guidelines: What they are and why they exist
In Australia, prescription medicines are not able to be advertised to the general public. These rules also extend to products that are ‘unregistered’, meaning they need special approval to be prescribed. Medical cannabis is an unregistered medicine.
That’s something that a lot of people like about Australia, you don’t see ads for antidepressants on TV and that kind of thing.
Companies, however, are allowed to provide information and resources about medicines to healthcare professionals. Accurate educational content is allowed to be made available to the general public. This can include information about medical conditions and health and wellness but the information must tread a very fine line, providing accurate and unbiased information and information that can not be perceived to be promoting prescription medicine or a specific product.
Why these rules exist

These rules were put in place to help protect the general public. Consumers and patients are not medical doctors. They’re not trained to understand or distinguish between accurate and inaccurate information on medicines. The idea behind the rules is that they allow doctors to access information about drugs and therapies so that they can learn all the relevant information. Doctors are then able to talk to patients about the medication or therapies and educate the patient directly with the correct information.
The bottom line is that the goal of these rules is to protect the general public. As you read through this article, think about how you can learn more about cannabis and those prescribing your medication in order to make the best decisions for your health and wellbeing.
Permitted & prohibited education and advertising
Permitted education and advertising
As already mentioned, the TGA does allow individuals and companies to provide some information to the general public. The guidelines allow for the provision of accurate, research-based (and cited), balanced (unbiased) information that doesn’t promote a product.
The problem is that there is a big grey area and many types of information which are considered to be advertising, are not permitted.
A honahlee example of education
The example we’ll use is our cannabis and anxiety article. In order to meet the TGA criteria, we cover various points in the article which, in theory, make it education. We have:
- A definition of anxiety and diagnosis
- Traditional treatments and side effects (balanced)
- Cannabis treatments and side effects (balanced)
- Research related to cannabis and anxiety (factual)
- Disclaimers that state cannabis needs more research (balanced)
- Disclaimers that state the TGA regulates cannabis
This allows our content to present a holistic view of these cannabis-related topics allowing the reader to learn and make an informed decision.
Prohibited education and advertising
The definition of advertising, according to the TGA guidelines, is any representation that is intended, whether directly or indirectly, to promote the use or supply of medical cannabis. It doesn’t actually matter what the intent of the information is. The guidelines state, “The intention is gauged not by what the person responsible for the content intends, but rather what the reasonable consumer views as being intended by the content.”
The problem with this method is that it is really just gauged by someone at the TGA as to whether they believe that the average consumer might read it and think that cannabis could be right for me.
That’s a very restrictive interpretation of the regulations. I don’t think that’s the intent of the regulations necessarily, but that’s the kind of scope that the TGA is playing with.
Examples of what’s not permitted
A few basic and obvious things that are not allowed are:
- Having images of medical cannabis products on your website.
- Promoting a specific product on social media.
- Providing patients special benefits (products or consults) for purchasing shares in your company
- Doctors or members of clinics actively participating in social media groups to promote themselves or their practices.
Points two through four above show a clear conflict of interest in the way a business operates. When these healthcare professionals or companies participate in activities like this, they have both direct and indirect impacts on patients. For example, if a doctor is promoting a single product such as a CBD tablet, specific oil or vape liquid, the odds are that they have some sort of commercial interest in providing that product.
As a patient or consumer, you must be aware of these types of conflicts of interest to make sure you’re not being pressured into or sold a product that may not be the best possible product for you or your condition.
How the guidelines impact patients

Because the guidelines aren’t being enforced by the TGA, the impact of these stringent regulations on patients is limited, but not non-existent.
Relating back to points two through four in the previous section. Those prohibited activities show a clear conflict of interest in the way an individual or business operates. When healthcare professionals or companies participate in activities like those, they have both direct and indirect impacts on patients. For example, if a doctor is promoting a single product such as a CBD tablet, specific oil or vape liquid, the odds are that they have some sort of commercial interest in providing that product.
As a patient or consumer, you must be aware of these types of conflicts of interest to make sure you’re not being pressured into or sold a product that may not be the best possible product for you or your condition.
The companies and doctors that are playing by the rules and working within the guidelines are also the ones that are most likely to have the patient at the forefront of their business. They are the ones that are more likely to be providing the public more accurate and unbiased information. And if they’re playing by the rules, you as a patient, are less likely to find them.
The other issue with the guidelines is that in many ways the can prohibit education which patients and consumers desperately need. While the TGA can’t regulate information from overseas, most of that overseas information is not relevant to the Australian medical cannabis market.
The takeaway here is to make sure you engage with your doctor and ask questions. The following questions are going to be the most important for you:
- Why have you chosen this product for me?
- Why have you chosen a compounded product over a branded SAS product?
- Do you have any financial connection to the product you’re prescribing?
- Are there similar but cheaper products to the one you’re prescribing?
- Are there more expensive products that you’ve found more effective?
How the guidelines impact the industry
One of the benefits of these guidelines is that many of the legal medical cannabis product companies have helped to educate doctors. Cannabis has become a much more popular treatment over the past couple of years. It’s important that our GPs and other healthcare professionals are informed about cannabis as a possible treatment for their patients. In an ideal world, the need for cannabis clinics would disappear because most of the doctors in Australia were educated and capable of providing good medical cannabis treatment.
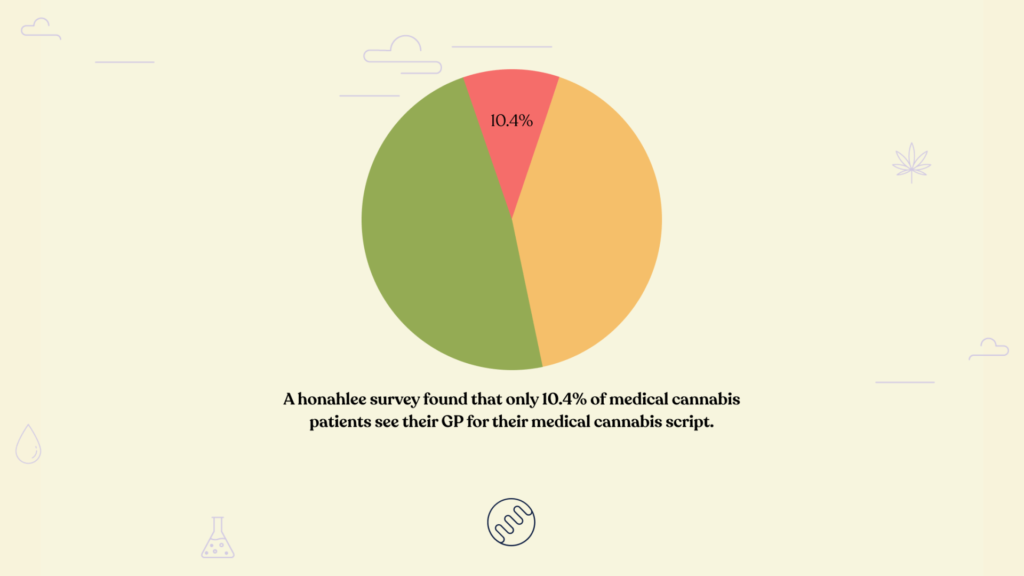
The other way that the current guidelines may impact the cannabis industry is possibly by affecting the success of some of the clinics and product companies. There are currently about 150 medical cannabis products available in Australia and about 30,000 active medical cannabis patients. The products that are most often prescribed aren’t always the products with the best cannabinoid profile, the most effective or even the cheapest. Quite often these products are the ones that are the best known.
So when you see a product being pushed on a social channel or on a website, ask yourself why.
The TGA’s enforcement of the guidelines
How the TGA finds regulatory breaches
Most of the time the TGA is made aware of breaches because a complaint has been made to the TGA. Recently, the TGA also asked for the industry to help them find companies or individuals not playing by the rules and for the industry to help counter misleading information. The TGA was then quoted saying that in countering misleading information, companies or individuals may fall in breach of the advertising guidelines.
Over the past two years, the TGA has received about 400 advertising complaints relating to medical cannabis or medical cannabis services. And while they’ve had so many complaints, it’s rare that the TGA actually meets its own target time for addressing these issues, whether they are low, medium or high priority.
Unfortunately, it doesn’t seem that the TGA does much regulating without assistance and even with assistance isn’t able to do the job they’ve set out to do.
TGA regulatory process
On the first instance, the TGA attempts to contact that organisation in breach of the guidelines and informs them that they have made a breach. The TGA requests that they remove or modify whatever information is in breach.
Depending on how that organisation responds, the TGA will engage in a dialogue on how to meet compliance.
If the organisation complies with the TGA guidelines then the case is closed. If the TGA can’t get in touch with the organisation responsible for a breach, or the organisation or individual is reluctant to change their behaviour, the TGA has various tools at their disposal.
Normally they’d start by issuing a cease and desist letter. From there, penalties can include fines or criminal charges. To date, there are no known cases of fines for those in breach of medical cannabis advertising guidelines even though there are very clear repeat offenders.
Why it’s not working
There are currently a number of TGA advertising guidelines breaches from cannabis clinics and product companies. These breaches can be found via a simple google or review of social media. If multiple industry players are breaking the rules and no one is actually being stopped, why would you be the first to actually comply? Would you put your business at risk when you know that the regulator isn’t going to do much?
Imagine You get a letter from the TGA saying, ‘Hey, we’ve noticed that information on your website could be construed as advertising or promoting a product. You need to fix that up.
You’d sit there and go, ‘Well, you know, everyone else in my sector is doing exactly the same thing. Why should I be the first one to put down my gun?’ I think that’s probably a situation that a lot of people are finding themselves in at the moment.
Should cannabis be treated like a pharmaceutical
Medical cannabis didn’t get legalised in Australia because a critical mass of doctors stood up and said, “I want the right to prescribe this product to my patient because I think it’s going to be effective. It was a grassroots movement of patients and carers who, despite not being able to legally access cannabis for medical purposes, were still accessing it and using it for medical purposes. They found that it was effective and mounted a very successful campaign to change the laws in Australia to allow medical cannabis, to be prescribed.
Medical cannabis is quite different from other medications because we are in a world where a very large number of people are already using cannabis to self medicate illegally via the green market. Many of these people have spent a lot of time learning about how it works, why it works and what kinds of products might be best for them. These patients then bring that information to their doctor to get legal medication. Most medications go the other way – doctors tell the patient all about the medication.
But this process probably isn’t right for the medical space in the long term and it presents unique challenges. There is so much information and misinformation on the internet about cannabis. Quite often this information, in Australia, falls into the territory of advertising. This usually happens when an individual or company talks about specific products or types of cannabis for specific conditions.
We do know that CBD and THC have both unique and cross over side effects and benefits. There is very limited evidence that once specific cannabis formulation or delivery method actually treats specific conditions better than another, unlike mainstream pharmacological treatments.
Do the guidelines work, or need to change?
The answer is probably that they need to change in some ways, but they are not completely wrong either.
I think it’s fine to prevent companies from advertising their products directly to patients. I think advertising products is inappropriate. We don’t do it with any other medicine. We shouldn’t allow that to happen with cannabis medicines.
Educational materials, however, are a tricky area. Patients and consumers need more materials that are designed to educate and inform. Most importantly, we need more high-quality information that people can trust and that counters some of the misinformation. The problem with the current regulations is that they are not clear.
The TGA often talks about the context of the content. They look at each individual part and combine them to decide whether it’s advertising or not. While the TGA isn’t strongly enforcing this, people will continue to find the incorrect and misleading information. If they do start enforcing rules that are so unclear, this could really negatively impact both patients and the industry overall by stamping out a majority of cannabis information.
At the end of the day, it comes down to what the TGA believes an average person will think when reading your website or whatever it is.
What can be done?
It’s pretty clear that product suppliers, clinics and doctors should not be able to directly advertise to the public. It’s also clear that specific products should not be directly advertised to the public. Finally, it’s clear that the conflicts of interest, where doctors or clinics have financial incentives to provide specific products to patients should not be allowed.
But, that doesn’t mean that the public shouldn’t know what’s out there. Australians need to know what products exist. They need to know where they can get advice from a medical professional. Australians also need to know about cannabis more generally, including the benefits, side effects, contraindications, delivery methods and much more.
Maybe the solution is not necessarily to change the guidelines drastically, but instead, making sure that certain educational websites, companies or government hubs get some sort of tick of approval. Maybe there’s a way to actually create a defined structure for what is considered education.
Conclusion
In many ways, this problem is similar to the question of legalisation vs prohibition. Just as people are always going to access cannabis, people are always going to access information about cannabis. The decision that the regulators need to make is how to best manage the access of information.
Removing access to all information, as the guidelines attempt to do now, is definitely not the best solution for patients or industry. The guidelines must allow for education while stopping individuals and companies who are purely out to make money.
Until the time where the TGA actually engages with both industry and the public on this topic, the hope is that they will just begin to tackle the biggest breaches of these guidelines. Doing so would create a level playing field for industry, but more importantly a much safer and trustable space for medical cannabis patients.



